H2S04, commonly known as sulfuric acid, is one of the most vital chemicals in the world, playing a crucial role in various industrial processes and applications. Its significance cannot be overstated, as it is utilized in the production of fertilizers, explosives, and even in petroleum refining. This article will delve into the properties, uses, safety measures, and environmental impacts of H2S04, providing a comprehensive overview for readers who seek to understand this essential compound better.
As the backbone of many chemical reactions, sulfuric acid's versatility makes it indispensable across multiple sectors. The production of sulfuric acid is a testament to its demand, with millions of tons produced annually. In this article, we will explore the various aspects of H2S04, including its chemical properties, industrial applications, and the necessary precautions that must be taken when handling it.
In addition, understanding the environmental implications of H2S04 and the regulations surrounding its use is crucial for both industries and individuals. This article aims to provide expert insights and reliable information to ensure that readers are well-informed about H2S04 and its impact on our lives.
Table of Contents
- What is H2S04?
- Properties of H2S04
- Industrial Applications of H2S04
- Safety Precautions When Handling H2S04
- Environmental Impact of H2S04
- Legal Regulations on H2S04
- Summary and Takeaways
- Conclusion
What is H2S04?
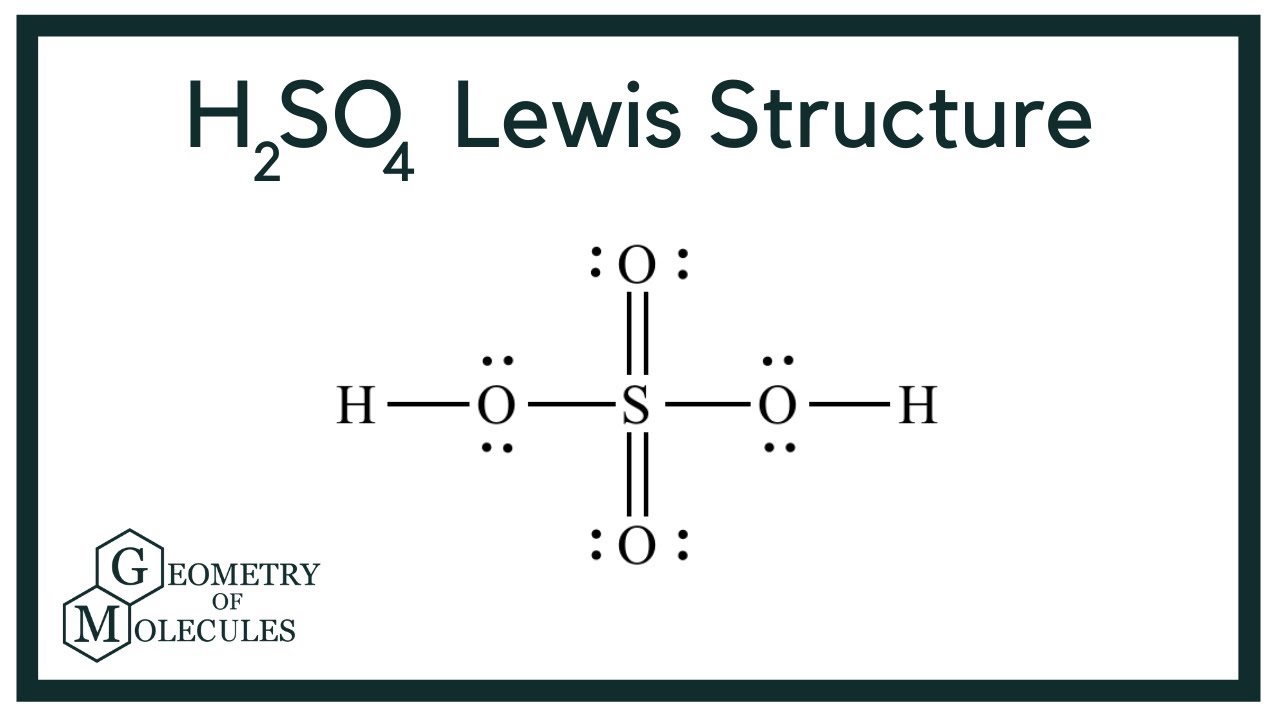
H2S04, or sulfuric acid, is a colorless, odorless, and highly corrosive liquid that is soluble in water. It is composed of two hydrogen atoms, one sulfur atom, and four oxygen atoms. Sulfuric acid is classified as a strong acid due to its ability to completely dissociate in water, releasing hydrogen ions, which makes it a powerful reagent in various chemical reactions.
Chemical Structure and Composition
The chemical formula H2S04 signifies the presence of two hydrogen (H) atoms, one sulfur (S) atom, and four oxygen (O) atoms. The molecular weight of sulfuric acid is approximately 98.08 g/mol, which contributes to its density and corrosiveness.
Physical Properties
- Appearance: Colorless liquid
- Odor: Odorless
- Density: 1.84 g/cm³
- Boiling Point: 337 °C (639 °F)
- Melting Point: 10 °C (50 °F)
Properties of H2S04
Understanding the properties of H2S04 is essential, as they determine how it interacts with other substances. The most notable properties include its strong acidity, hygroscopic nature, and ability to act as a dehydrating agent.
Acidity
H2S04 is one of the strongest acids, making it highly effective in neutralizing bases and reacting with various metals. Its ability to release protons in aqueous solutions contributes to its high reactivity.
Hygroscopic Nature
Sulfuric acid is hygroscopic, meaning it can absorb moisture from the air. This property makes it a useful drying agent in chemical processes. However, it also poses risks when exposed to humid environments.
Industrial Applications of H2S04
The versatility of H2S04 is evident in its numerous applications across various industries. Here are some of the primary uses of sulfuric acid:
Fertilizer Production
One of the largest uses of H2S04 is in the production of phosphate fertilizers. It reacts with phosphate rock to produce phosphoric acid, which is essential for plant growth.
Pulp and Paper Industry
Sulfuric acid is used in the pulp and paper industry for the production of wood pulp from cellulose fibers. It helps in the breakdown of lignin, facilitating the separation of cellulose.
Petroleum Refining
In the petroleum industry, sulfuric acid is employed in the alkylation process, which combines light hydrocarbons to produce high-octane gasoline.
Manufacturing of Chemicals
H2S04 is a key reagent in the manufacturing of various chemicals, including hydrochloric acid, nitric acid, and sulfate salts.
Safety Precautions When Handling H2S04
Given its corrosive nature, safety precautions are paramount when handling sulfuric acid. Here are essential safety measures:
- Wear appropriate personal protective equipment (PPE), including gloves, goggles, and face shields.
- Ensure proper ventilation in the working area to avoid inhalation of fumes.
- Store H2S04 in a cool, dry place away from incompatible materials.
- Have neutralizing agents, such as sodium bicarbonate, readily available in case of spills.
Environmental Impact of H2S04
The production and use of sulfuric acid can have significant environmental implications. When released into the environment, H2S04 can contribute to acid rain, which adversely affects ecosystems, soil, and water sources.
Acid Rain Formation
The sulfur dioxide (SO2) released during sulfuric acid production can react with moisture in the atmosphere to form sulfuric acid, leading to acid rain.
Regulatory Measures
To mitigate the environmental impact, numerous regulations are in place to control sulfur emissions and promote safer handling practices. Industries must adhere to these regulations to minimize their ecological footprint.
Legal Regulations on H2S04
Various legal frameworks govern the production, distribution, and use of H2S04. These regulations aim to ensure safety and environmental protection.
- The Resource Conservation and Recovery Act (RCRA) regulates the management of hazardous waste, including sulfuric acid.
- Occupational Safety and Health Administration (OSHA) standards dictate safety practices for workers handling hazardous materials.
Summary and Takeaways
In summary, H2S04, or sulfuric acid, is a fundamental chemical in many industrial processes, particularly in fertilizer production and petroleum refining. Its properties, such as high acidity and hygroscopic nature, contribute to its versatility but also necessitate strict safety measures during handling. Understanding the environmental impact and legal regulations surrounding H2S04 is crucial for responsible usage.
Conclusion
In conclusion, sulfuric acid is a powerful and essential compound in various industries. Being informed about its properties, applications, and safety precautions can help individuals and organizations utilize H2S04 responsibly. If you found this article helpful, feel free to leave a comment, share it with others, or explore more articles on our site for additional insights.
Article Recommendations