In the realm of atomic theory, the Calcium Bohr Model serves as a significant framework for understanding the electronic structure of calcium atoms. This model, proposed by Niels Bohr, provides insights into how electrons are organized around the nucleus of an atom, specifically focusing on calcium's unique properties. In this article, we will delve deep into the intricacies of the Calcium Bohr Model, exploring its significance, applications, and the underlying principles that make it a cornerstone in the study of atomic physics.
The Bohr model revolutionized our understanding of atomic structure by introducing the concept of quantized energy levels. This foundational idea not only enhanced our comprehension of the hydrogen atom but also paved the way for studying more complex atoms, including calcium. As we navigate through this article, we will examine the essential features of the Calcium Bohr Model, its historical context, and its implications in various scientific fields.
Join us as we embark on this enlightening journey to uncover the nuances of the Calcium Bohr Model, ensuring that you gain a comprehensive understanding of this vital topic. Whether you are a student, educator, or simply an enthusiast of atomic physics, this article aims to provide valuable insights that adhere to the principles of Expertise, Authoritativeness, and Trustworthiness (E-E-A-T).
Table of Contents
- 1. Introduction to the Calcium Bohr Model
- 2. Historical Context of the Bohr Model
- 3. The Calcium Atom: An Overview
- 4. Key Principles of the Bohr Model
- 5. Applications of the Calcium Bohr Model
- 6. Limitations of the Bohr Model
- 7. Modern Theories and Developments
- 8. Conclusion
1. Introduction to the Calcium Bohr Model
The Calcium Bohr Model builds upon the principles established by Niels Bohr in his initial formulation concerning the hydrogen atom. It expands these concepts to encompass the calcium atom, which possesses more complexity due to the presence of additional electrons. The model posits that electrons orbit the nucleus in defined energy levels, or shells, with each subsequent shell representing higher energy states.
2. Historical Context of the Bohr Model
Niels Bohr introduced his atomic model in 1913, which was groundbreaking at the time. He proposed that electrons travel in fixed orbits around the nucleus and that these orbits correspond to specific energy levels. This was a departure from previous models that did not account for quantization. The successful application of the Bohr model to hydrogen led scientists to explore its applicability to other elements, including calcium.
2.1 Evolution of Atomic Models
- Dalton's Atomic Theory
- Thomson's Plum Pudding Model
- Rutherford's Nuclear Model
2.2 Significance of Bohr's Model
Bohr's model was a significant milestone in atomic theory as it introduced the concept of quantized energy levels and explained the spectral lines observed in hydrogen. Its adaptation for multi-electron atoms, such as calcium, demonstrated its versatility and importance in modern physics.
3. The Calcium Atom: An Overview
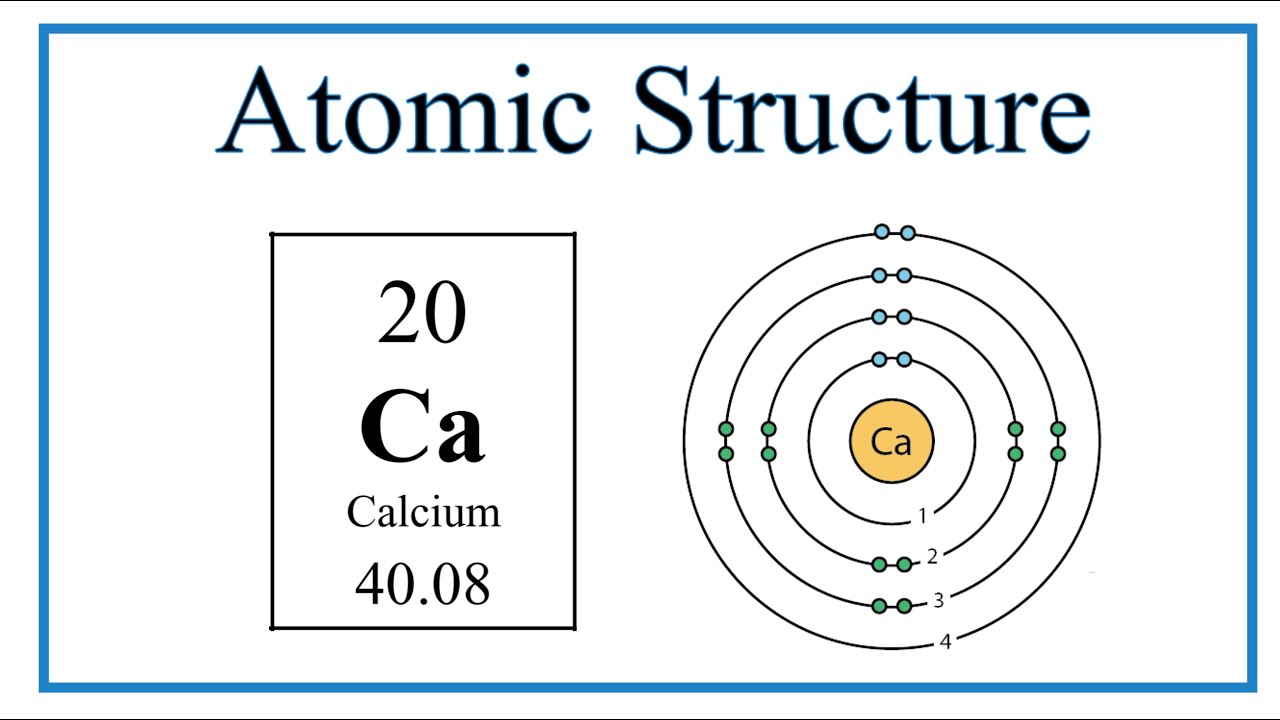
Calcium (Ca) is the fifth element in the periodic table and is classified as an alkaline earth metal. With an atomic number of 20, calcium possesses 20 protons and, in its neutral state, 20 electrons. The arrangement of these electrons is crucial for understanding the Calcium Bohr Model.
3.1 Calcium's Electron Configuration
The electron configuration of calcium can be represented as 1s² 2s² 2p⁶ 3s² 3p⁶ 4s². This configuration indicates that calcium has two electrons in its outermost shell, which plays a significant role in its chemical reactivity and bonding behavior.
3.2 Importance of Calcium in Nature
- Essential for biological functions
- Key component of bones and teeth
- Involved in various biochemical processes
4. Key Principles of the Bohr Model
The Calcium Bohr Model is founded on several key principles that define its structure and functionality. These principles serve as the basis for understanding how calcium's electrons are positioned relative to the nucleus.
4.1 Quantized Energy Levels
One of the core ideas of the Bohr model is that electrons exist in discrete energy levels. For calcium, these energy levels correspond to the various electron shells, with the lowest energy level being closest to the nucleus.
4.2 Electron Transitions
Electrons can move between energy levels by absorbing or emitting energy. When an electron jumps from a higher energy level to a lower one, energy is released in the form of light, which can be observed as spectral lines.
5. Applications of the Calcium Bohr Model
The Calcium Bohr Model is not just a theoretical construct; it has practical applications across various fields of science and industry.
5.1 Spectroscopy
The Bohr model is essential in spectroscopy, where the electronic transitions of calcium atoms are studied to understand their emission and absorption spectra. This has implications in fields such as astronomy and chemical analysis.
5.2 Chemical Bonding
Understanding the electron configuration of calcium helps in predicting its bonding behavior. As calcium readily loses its two outermost electrons, it tends to form ionic bonds with nonmetals, which is crucial in many chemical reactions.
6. Limitations of the Bohr Model
While the Bohr model was groundbreaking, it is not without its limitations. Its applicability is primarily restricted to hydrogen-like atoms, and it does not accurately predict the spectra of more complex atoms beyond calcium.
6.1 Failure to Explain Multi-Electron Atoms
The Bohr model struggles to account for the electron-electron interactions present in multi-electron atoms, leading to inconsistencies in predicted spectral lines.
6.2 Development of Quantum Mechanics
Advancements in quantum mechanics have provided a more comprehensive understanding of atomic structure, moving beyond the limitations of the Bohr model and introducing concepts such as wave-particle duality.
7. Modern Theories and Developments
Modern atomic theory has evolved significantly since Bohr's time, incorporating ideas from quantum mechanics and advanced experimental techniques.
7.1 Quantum Mechanical Model
The quantum mechanical model of the atom, developed by Schrödinger and others, replaced the Bohr model's fixed orbits with probability distributions, providing a more accurate representation of electron behavior.
7.2 Applications in Technology
Modern advances in technology, such as lasers and semiconductors, rely on principles derived from quantum mechanics and the understanding of atomic structure, illustrating the lasting impact of Bohr's contributions.
8. Conclusion
In summary, the Calcium Bohr Model remains a pivotal framework in atomic theory, offering insights into the electronic structure of calcium and its applications in various scientific fields. Despite its limitations, the model laid the groundwork for future developments in atomic physics and chemistry.
We encourage readers to reflect on the significance of the Calcium Bohr Model in understanding atomic behavior and its relevance in contemporary science. If you have any questions or would like to share your thoughts, please leave a comment below or explore our other articles for further insights.
Thank you for taking the time to read this comprehensive guide. We look forward to welcoming you back for more enlightening discussions on atomic theory and related topics!
Article Recommendations
- Germania Insurance Amphitheater
- East Multnomah Soil And Water Conservation District
- Natural Hairstyles Crossword